Egypt Begins Production of Coronavirus Medication Favipiravir
The Egyptian Drug Authority has given its approval for 61 local drug companies to produce Favipiravir, an antiviral medication for the Coronavirus.
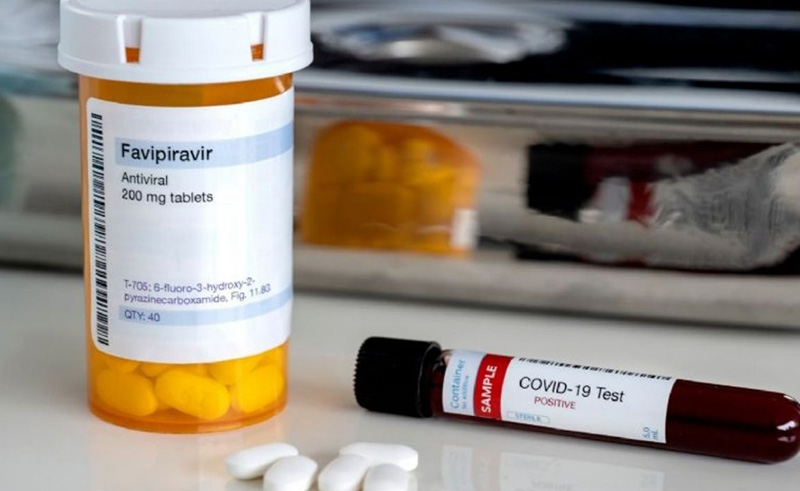
Following several clinical trials, the Egyptian Drug Authority (EDA) has given its approval for 61 local drug companies to begin producing the antiviral medication Favipiravir, which is hoped to treat mild to moderate cases of COVID-19. These companies are now allowed to start the technical, logistical and administrative procedures to register the drug.
Favipiravir is a Japanese drug that has been used in the past to treat novel influenza (strains that lead to more severe diseases), in addition to various other viral infections. With the spread of COVID-19 on a global scale, the drug has undergone several clinical tests in the hopes that it could serve as a potential treatment for the virus, and has so far demonstrated positive results.
Four undisclosed companies have already completed procedures for the registration process, with only one company being granted registration so far. Additionally, the EDA has approved applications submitted by 27 different drug companies to produce Remdesivir, which can be used to treat moderate to severe cases of Coronavirus.
























